Đào tạo & nghiên cứu
Evaluation of the Immune Response in Vaccinated and SARS – CoV – 2 Infected Individuals in Vietnam by Detection of Neutralizing Antibody Using ELISA
D.K.Tran[1,2], H.T.Pham[1]*, Q.D.Ha[1,2], N.H.Tran[1,2], V.H.Pham[1,3]
[1] Vietnam Research and Development Institute of Clinical Microbiolgy, Vietnam
[2] Nam Khoa Biotek Co. Ltd., Vietnam
[3] Phan Chau Trinh University, Vietnam
1. Background
With the increasing diversity of new strains, the emergence of new vaccines, as well as varying access conditions to vaccines among countries, raises questions about the effectiveness of vaccine immune responses, post-immunity, or the strategy to diversify the immune response status within the community. To address these challenges, immune measurement tools are one of the feasible solutions to provide a scientific, practical, and meaningful perspective in assessing the individual immunity levels in the context of comprehensive SARS-CoV-2 prevention solutions.
2. Aims of the study
We pursued two primary objectives: (1) Analyzing the correlation between neutralizing antibody levels and the amount of antibodies against the S protein (2) to evaluating the immune response of neutralizing antibodies in different demographic groups who have completed vaccination corresponding to the vaccine groups used in Vietnam. Analyzing the influence of factors such as gender, age, time between two doses, and time after vaccination on the formation of neutralizing antibodies (3) to evaluating the neutralizing antibodies level response in individuals who have received booster shots across different vaccine groups in Vietnam (4) Analyzing the level of neutralizing antibodies in individuals infected with SARS-CoV-2 along with vaccination history.
3. Materials and methods
Blood samples were collected from voluntary participants in the research with a vaccination history (2 doses, 3 doses) or a history of SARS-CoV-2 infection from November 2021 to November 2022 in Vietnam. The blood samples were then centrifuged to obtain plasma, which was used for ELISA experiments to detect specific antibodies against the S protein by using The Invitrogen Human SARS-CoV-2 Spike (trimer) Ig Total ELISA Kit, and the Invitrogen SARS-CoV-2 Neutralizing Antibody ELISA Kit was used for neutralizing antibody expression. The optical density (OD) values obtained from the raw ELISA data were used to calculate the neutralizing antibody levels (% – Neutralization) and the antibody load against the S protein (IU/mL). Statistical operations, tests, and evaluations were conducted using SPSS 25 software to assess correlations, conduct ranking evaluations for test parameters, including descriptive statistics, Pearson correlation analysis, linear regression, Independent Sample T – Test, and One – way ANOVA for mean difference analysis.
4. Results and discussions
The total number of samples in the study reached 1000. Among them, the group of individuals who received 2 doses consisted of 330 people (Age: 41.25 ± 15.85), the group receiving booster shots had 339 individuals (Age: 45.55 ± 18.59), and the group with a history of SARS-CoV-2 infection comprised 331 individuals (Age: 38.08 ± 13.79). Observing a correlation between the concentration of antibodies against the S protein and the level of neutralizing antibody expression (r (Pearson) = 0.501, p < 0.0001) and assessing the feasibility of using the ELISA test for neutralizing antibodies in evaluating the immune response to SARS-CoV-2. The immune response in individuals receiving two doses of mRNA vaccines, such as MD–MD (Moderna (mRNA – 1273)), achieved 89.52%, and PF–PF (Pfizer (BNT162b2)) reached 78.27%, which is significantly higher than vector vaccines AZ–AZ ( AstraZeneca (ChAdOx1 )) and inactivated vaccine VC–VC (VeroCell (BBIBP-CorV)), which reached 48.27% and 27.06%, respectively. Heterologous vaccination of vector and mRNA vaccine, as seen in AZ–MD at 78.75% and AZ–PF at 73.55%, showed comparable results to the group receiving two doses of mRNA vaccines and higher than the pure AZ–AZ group. The study showed a negative correlation neutralizing antibody response and age in most vaccine groups, except for the PF–PF group (p < 0.0001). The interval between the first and second doses was found to have no significant link with neutralizing antibody levels. There was a dependence on the level of neutralizing antibody expression and the time after vaccination in the AZ–AZ, PF–PF groups, and the mixed-vaccine group (p (AZ-AZ) < 0.001, p (PF-PF) = 0.015, p (AZ-PF) < 0.001). Gender differences in neutralizing antibody levels were noted in the VC–VC vaccine group (p = 0.02), with no significant differences observed in other groups. A booster shot was reported to significantly increase neutralizing antibodies compared to the previous two doses. We observed a substantial rise in neutralizing antibody capacity among participants who received PF as booster vaccine, with a mean antibody level of 84.77%, compared to those receiving AZ vaccine, with a mean level of 66.35% (p < 0.0001). In detail, the results were outstanding in the AZ-AZ-PF group, exhibiting the median of neutralizing antibody level of 79.95%, and the VC-VC-PF group, which displayed a level of 91.02%. The mixed group of AZ-MD-PF achieved a value of 93.37% and AZ-PF-PF reached 85.39%, both surpassing the homologous booster AZ-AZ-AZ (64.32%), as well as the group injected with AZ-MD-AZ (67.49%) or AZ-PF-AZ group (63.4%). The level of neutralizing antibodies in convalescent participants without vaccination was low at 40.42% when compared to infected individuals who had a history of receiving at least one or two doses of vaccine, which reached values of 84.03% (p < 0.0001) and 86.65% (p < 0.0001), respectively.
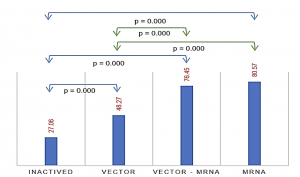
Graph 1: The level of neutralizing antibody expression according to the type of vaccine (One-way ANOVA Test)
|
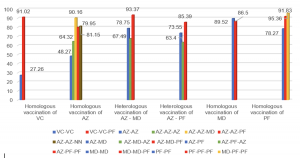
Graph 2: The level of neutralizing antibodies before and after receiving booster shots among groups of individuals who have received two doses of the vaccine.
|
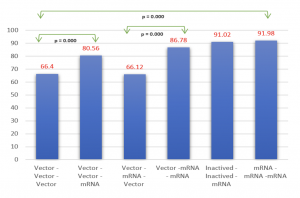
Graph 3: The level of neutralizing antibodies across vaccine recipient groups based on the nature of the vaccine technology. |
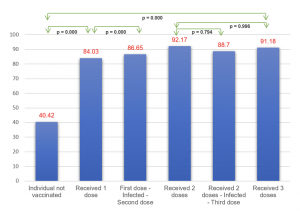
Graph 4: The average difference in neutralizing antibody levels among individuals infected with SARS-CoV-2 with different vaccination histories.
|
5. Conclusions
Our study is one of the first to analyze post-immune responses and booster vaccination across different COVID-19 vaccine groups circulating in Vietnam. The neutralizing antibody response in individuals who completed the vaccination course shows effectiveness when receiving mRNA vaccines compared to vector vaccines and inactivated vaccines. The heterologous vaccination yields a neutralizing antibody response at levels equivalent to mRNA vaccines and higher than the homologous vaccination groups of other types. The booster shot significantly increases the level of neutralizing antibodies in the AZ and VC vaccine groups. In the mixed-vaccine group or pure mRNA vaccination, the increase in antibody levels is negligible for the third dose. However, booster shots are deemed necessary in the extended vaccination strategy to enhance immunity and compensate for the decline over time after completing the initial vaccination. It is advisable to administer at least one dose of the vaccine to everyone to limit the severe impact of natural SARS-CoV-2 virus infection. The results indicate that individuals who have received at least one dose and subsequently get infected have significantly higher antibody levels compared to those who have never been vaccinated. In individuals who have been previously infected with the virus and have completed the full vaccination series, the booster shot is unnecessary.


